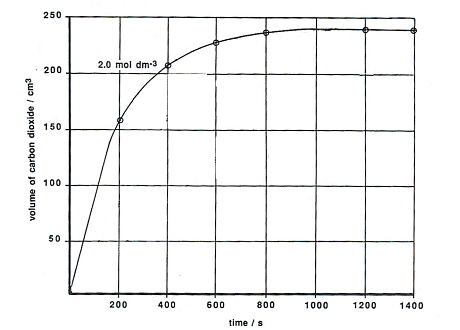
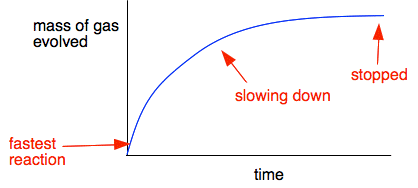
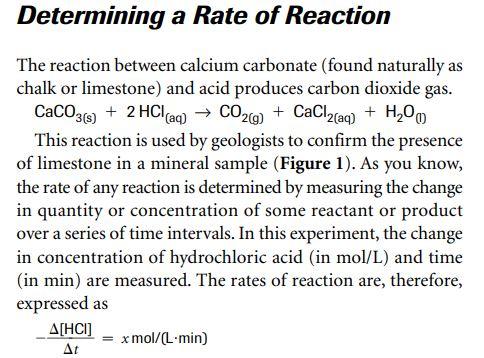
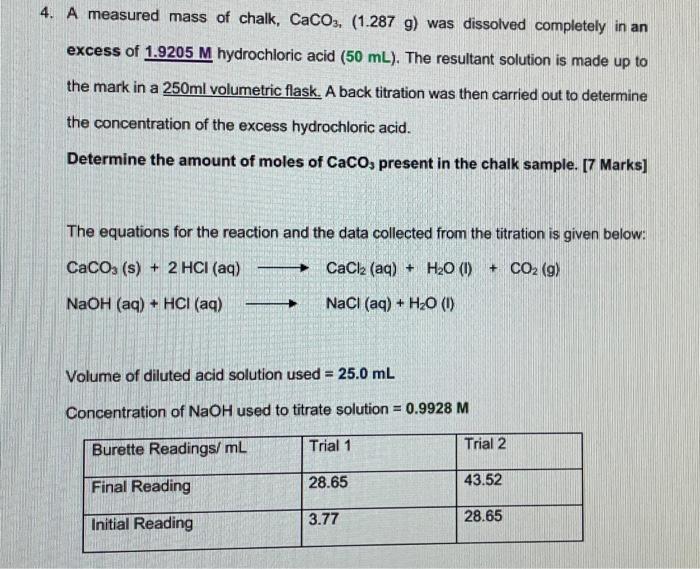
30. Calcium carbonate reacts with HCl to give CaCl,and CO2 according to the reaction:+ 2HCI(aq) CaCl, (aq) + CO2(g)CaCO3(s)H20(I) What mass of 20

Question Video: Writing a Net Ionic Equation for the Reaction of Solid Calcium Carbonate with a Hydrochloric Acid Solution | Nagwa
What is the balanced net-ionic equation for the gas producing reaction between hydrobromic acid and solid calcium carbonate? - Quora

Pure calcium carbonate and dilute hydrochloric acid are reacted and 2 litres of carbon dioxide was collected 27°C and normal pressure. CaCO3 + 2HCl → CaCl2 + H2O + CO2 Calculate : (
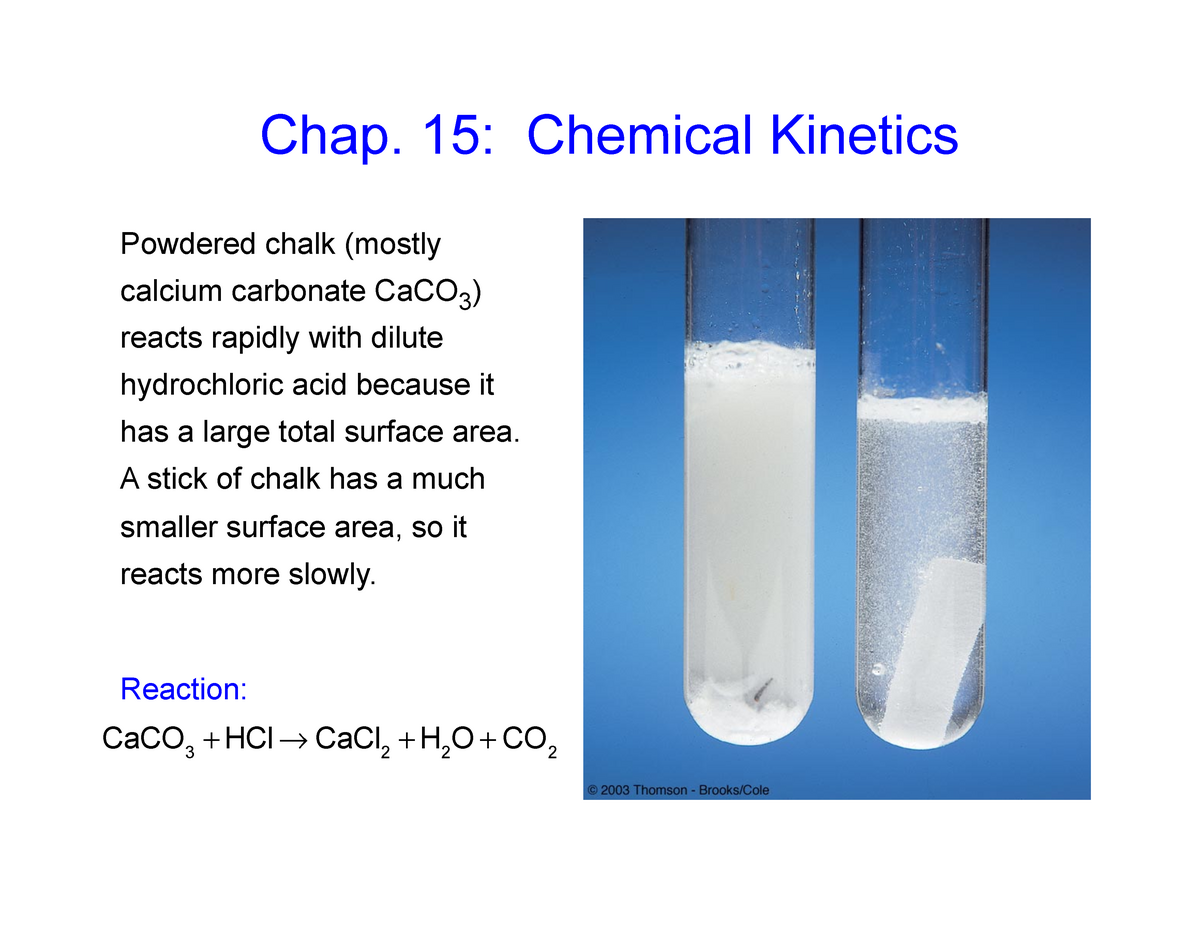
Chapter 15 slides - S1 - Lecture notes 15 - Chap. 15: Chemical Kinetics Powdered chalk (mostly - Studocu

hydrochloric acid and calcium carbinate limestone marble chemical reaction - LIACOS EDUCATIONAL MEDIA